Minggu, 18 Oktober 2009
Sabtu, 17 Oktober 2009
Sabtu, 03 Oktober 2009
Fisika Nuklir
Modern nuclear physics
A heavy nucleus can contain hundreds of nucleons which means that with some approximation it can be treated as a classical system, rather than a quantum-mechanical one. In the resulting liquid-drop model, the nucleus has an energy which arises partly from surface tension and partly from electrical repulsion of the protons. The liquid-drop model is able to reproduce many features of nuclei, including the general trend of binding energy with respect to mass number, as well as the phenomenon of nuclear fission.
Superimposed on this classical picture, however, are quantum-mechanical effects, which can be described using the nuclear shell model, developed in large part by Maria Goeppert-Mayer. Nuclei with certain numbers of neutrons and protons (the magic numbers 2, 8, 20, 28, 50, 82, 126, ...) are particularly stable, because their shells are filled.
Other more complicated models for the nucleus have also been proposed, such as the interacting boson model, in which pairs of neutrons and protons interact as bosons, analogously to Cooper pairs of electrons.
Much of current research in nuclear physics relates to the study of nuclei under extreme conditions such as high spin and excitation energy. Nuclei may also have extreme shapes (similar to that of Rugby balls) or extreme neutron-to-proton ratios. Experimenters can create such nuclei using artificially induced fusion or nucleon transfer reactions, employing ion beams from an accelerator. Beams with even higher energies can be used to create nuclei at very high temperatures, and there are signs that these experiments have produced a phase transition from normal nuclear matter to a new state, the quark-gluon plasma, in which the quarks mingle with one another, rather than being segregated in triplets as they are in neutrons and protons.
Eighty elements have at least one stable isotope never observed to decay, amounting to a total of about 255 stable isotopes. However, thousands of isotopes have been characterized that are unstable. These radioisotopes decay over time scales ranging from fractions of a second to weeks, years, or billions of years.
The stability of a nucleus is highest when it falls into a certain range or balance of composition of neutrons and protons; too few or too many neutrons may cause it to decay. For example, in beta decay a nitrogen-16 atom (7 protons, 9 neutrons) is converted to anoxygen-16 atom (8 protons, 8 neutrons) within a few seconds of being created. In this decay a neutron in the nitrogen nucleus is converted into a proton and an electron and an antineutrino by the weak nuclear force. The element is transmuted to another element in by acquiring the created proton.
In alpha decay the radioactive element decays by emitting a helium nucleus (2 protons and 2 neutrons), giving another element, plus helium-4. In many cases this process continues through several steps of this kind, including other types of decays, until a stable element is formed.
In gamma decay, a nucleus decays from an excited state into a lower energy state, by emitting a gamma ray. The element is not changed to another element in the process (no nuclear transmutation is involved).
Other more exotic decays are possible (see the main article). For example, in internal conversion decay, the energy from an excited nucleus may be used to eject one of the inner orbital electrons from the atom, in a process which produces high speed electrons, but is not beta decay, and (unlike beta decay) does not transmute one element to another.
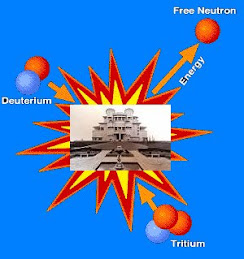
In nuclear fusion, two low mass nuclei come into very close contact with each other, so that the strong force fuses them. It requires a large amount of energy to overcome the repulsion between the nuclei for the strong or nuclear forces to produce this effect, therefore nuclear fusion can only take place at very high temperatures or high pressures. Once the process succeeds, a very large amount of energy is released and the combined nucleus assumes a lower energy level. The binding energy per nucleon increases with mass number up until nickel-62. Stars like the Sun are powered by the fusion of four protons into a helium nucleus, two positrons, and twoneutrinos. The uncontrolled fusion of hydrogen into helium is known as thermonuclear runaway. A frontier in current research at various institutions, for example the Joint European Torus (JET) and ITER, is the development of an economically viable method of using energy from a controlled fusion reaction.
Nuclear fission is the reverse process of fusion. For nuclei heavier than nickel-62 the binding energy per nucleon decreases with the mass number. It is therefore possible for energy to be released if a heavy nucleus breaks apart into two lighter ones.
The process of alpha decay is in essence a special type of spontaneous nuclear fission. This process produces a highly asymmetrical fission because the four particles which make up the alpha particle are especially tightly bound to each other, making production of this nucleus in fission particularly likely.
For certain of the heaviest nuclei which produce neutrons on fission, and which also easily absorb neutrons to initiate fission, a self-igniting type of neutron-initiated fission can be obtained, in a so-called chain reaction. Chain reactions were known in chemistry before physics, and in fact many familiar processes like fires and chemical explosions are chemical chain reactions. The fission or "nuclear" chain-reaction, using fission-produced neutrons, is the source of energy for nuclear power plants and fission type nuclear bombs, such as those detonated by the United States in Hiroshima and Nagasaki, Japan, at the end of World War II. Heavy nuclei such as uraniumand thorium may undergo spontaneous fission, but they are much more likely to undergo decay by alpha decay.
For a neutron-initiated chain-reaction to occur, there must be a critical mass of the element present in a certain space under certain conditions (these conditions slow and conserve neutrons for the reactions). There is one known example of a natural nuclear fission reactor, which was active in two regions of Oklo, Gabon, Africa, over 1.5 billion years ago. Measurements of natural neutrino emission have demonstrated that around half of the heat emanating from the Earth's core results from radioactive decay. However, it is not known if any of this results from fission chain-reactions.
According to the theory, as the Universe cooled after the big bang it eventually became possible for common subatomic particles as we know them (neutrons, protons and electrons) to exist. The most common particles created in the big bang which are still easily observable to us today were protons and electrons (in equal numbers). The protons would eventually form hydrogen atoms. Almost all the neutrons created in the Big Bang were absorbed into helium-4 in the first three minutes after the Big Bang, and this helium accounts for most of the helium in the universe today (see Big Bang nucleosynthesis).
Some fraction of elements beyond helium were created in the Big Bang, as the protons and neutrons collided with each other (lithium, beryllium, and perhaps some boron), but all of the "heavier elements" (heavier than carbon, element number 6) that we see today, were created inside of stars during a series of fusion stages, such as the proton-proton chain, the CNO cycle and the triple-alpha process. Progressively heavier elements are created during the evolution of a star.
Since the binding energy per nucleon peaks around iron, energy is only released in fusion processes occurring below this point. Since the creation of heavier nuclei by fusion costs energy, nature resorts to the process of neutron capture. Neutrons (due to their lack of charge) are readily absorbed by a nucleus. The heavy elements are created by either a slow neutron capture process (the so-called sprocess) or by the rapid, or r process. The s process occurs in thermally pulsing stars (called AGB, or asymptotic giant branch stars) and takes hundreds to thousands of years to reach the heaviest elements of lead and bismuth. The r process is thought to occur in supernova explosions because the conditions of high temperature, high neutron flux and ejected matter are present. These stellar conditions make the successive neutron captures very fast, involving very neutron-rich species which then beta-decay to heavier elements, especially at the so-called waiting points that correspond to more stable nuclides with closed neutron shells (magic numbers). The r process duration is typically in the range of a few seconds.
Kamis, 01 Oktober 2009
Fisika Nuklir
Nuclear physics is the field of physics that studies the building blocks and interactions of atomic nuclei. The most commonly known applications of nuclear physics are nuclear power generation and nuclear weapons technology, but the research has provided application in many fields, including those in nuclear medicine and magnetic resonance imaging, ion implantation in materials engineering, and radiocarbon dating in geology and archaeology.
The field of particle physics evolved out of nuclear physics and is typically taught in close association with nuclear physics.
Introduction to Applied Nuclear Physics

The Rutherford-Bohr model of the atom. (Courtesy ofEPA.)
Level:
Instructors:
Prof. Kim Molvig
Course Description
This course concentrates on the basic concepts of nuclear physics with emphasis on nuclear structure and radiation interactions with matter. Included: elementary quantum theory; nuclear forces; shell structure of the nucleus; alpha, beta, and gamma radioactive decays; interactions of nuclear radiations (charged particles, gammas, and neutrons) with matter; nuclear reactions; and fission and fusion.
The course is divided into three main sections:
- Quantum Mechanics Fundamentals
- Nuclear Structure and Nuclear Decays
- Interactions in Nuclear Matter and Nuclear Reactions
Calendar
| SES # | TOPICS | READINGS & HANDOUTS |
|---|---|---|
| 1 | Intro Lectures: Basic Nucleus Concepts | |
| 2 | Intro Lectures: Wave-Particle Duality & Historical Background | |
| 3 | Quantum Mechanics #1, #2
| Liboff 3.1-3.3 Postulates Handout |
| 4 | Quantum Mechanics #3, #4
| Liboff 4.1, 4.2, 4.3 Liboff 7.5, 7.6, 7.7 |
| 5 | Quantum Mechanics #5, #6
| Liboff 5.1-5.5 |
| 6 | Quantum Mechanics #7, #8
| Liboff 3.4, 3.5, 6.2 Liboff 9.1-9.3 |
| 7 | Quantum Mechanics #9
| |
| 8 | Mid-Term Exam | |
| 9 | Nuclear Structure #1, #2
| Krane 3 Krane 4 |
| 10 | Nuclear Structure #3, #4
| Krane 5 |
| 11 | Nuclear Structure #5 -Radioactive Decay, Alpha Decay | Krane 8 |
| 12 | Gamma Decay | Liboff 10.7 Krane 10 |
| 13 | Nuclear Interactions #1, - Charged Particle Interactions | Krane 9 Krane 7 |
| 14 | Beta Decay Nuclear Interactions #1, - Charged Particle Interactions (Cont'd) | Liboff 10.7 Krane 10 |
| 15 | Nuclear Interactions #2, #3
| Krane 12 |
| 16 | Nuclear Interactions #4, #5
| Krane 13 Krane 14 |
References
- ^ B. R. Martin (2006). Nuclear and Particle Physics. John Wiley & Sons, Ltd.. ISBN 0-470-01999-9.
- ^ Henri Becquerel (1896). "Sur les radiations émises par phosphorescence". Comptes Rendus 122: 420–421.
- ^ Philosophical Magazine (12, p 134-46)
- ^ Proc. Roy. Soc. July 17, 1908
- ^ Proc. Roy. Soc. A82 p 495-500
- ^ Proc. Roy. Soc. Feb. 1, 1910
- ^ W. Pauli, Nobel lecture, December 13, 1946.
- ^ "Alexandru Proca (1897-1955) and his equation of the massive vector boson field by Dorin N. Poenaru 1, 2 and Alexandru Calboreanu". http://dx.doi.org/10.1051/epn:2006504 (Europhysics News): 37 (5): 25–27.
- ^ G. A. Proca, Alexandre Proca.Oeuvre Scientifique Publiée, S.I.A.G., Rome, 1988.
- ^ C. Vuille, J. Ipser, J. Gallagher, “Einstein-Proca model, micro black holes, and naked singularities”, General Relativity and Gravitation,34 (2002), 689.
- ^ R. Scipioni, “Isomorphism between non-Riemannian gravity and Einstein-Proca-Weyl theories extended to a class of scalar gravity theories”, Class. Quantum Gravity., 16 (1999), 2471.
- ^ R. W. Tucker and C. Wang, C., “An Einstein-Proca-fluid model for dark matter gravitational interactions”, Nucl. Phys. B - Proc. suppl., 57(1997) 259.
- Nuclear Physics by Irving Kaplan 2nd edition, 1962 Addison-Wesley
- General Chemistry by Linus Pauling 1970 Dover Pub. ISBN 0-486-65622-5
- Introductory Nuclear Physics by Kenneth S. Krane Pub. Wiley
- N.D. Cook (2010). Models of the Atomic Nucleus (2nd ed.). Springer. pp. xvi & 324. ISBN 978-3-642-14736-4.