•P. E. Hodgson, E. Gadioli, E. GadioliErba, Introductory Nuclear
Physics (Oxford U. P., New York, 2000)
•J. M. Blatt& V. F. Weisskopf, Theoretical Nuclear Physics
•W. E. Meyerhof, Elements of Nuclear Physics
Isi
•pendahuluan
•sifat-sifat inti
•ketidak stabilan inti
•radioaktivitas
•model inti
•gaya nuklir/ interaksi kuat
•fisika partikel
•astrofisika nuklir
•akselerator dan detektor
•reaktor nuklir
1. Atomic Structure and the Periodic Table
According to the Bohr-Rutherford model of the atom, also called the “solar system model,”the atom consists of a central nucleus surrounded by electrons in orbits around the nucleus. The nucleus is very massive, and the electrons are very light. Both the electrons and the nucleus are very much smaller than the overall size of the atom (the electrons may actually have no size at all). Since the nucleus is positively charged and the electrons are negatively charged, the electrons are attracted toward the nucleus, like the planets are attracted toward the sun, and they orbit around the nucleus is somewhat the same way. While this model has deficiencies that we talk about later, it is sufficient for our present purposes.

History
The history of nuclear physics as a discipline distinct from atomic physics starts with the discovery of radioactivity by Henri Becquerel in 1896,[1] while investigating phosphorescence in uranium salts.[2] The discovery of the electron by J. J. Thomson a year later was an indication that the atom had internal structure. At the turn of the 20th century the accepted model of the atom was J. J. Thomson's plum pudding model in which the atom was a large positively charged ball with small negatively charged electrons embedded inside of it. By the turn of the century physicists had also discovered three types of radiation emanating from atoms, which they named alpha, beta, and gamma radiation. Experiments in 1911 by Lise Meitner and Otto Hahn, and by James Chadwick in 1914 discovered that the beta decay spectrum was continuous rather than discrete. That is, electrons were ejected from the atom with a range of energies, rather than the discrete amounts of energies that were observed in gamma and alpha decays. This was a problem for nuclear physics at the time, because it indicated that energy was not conserved in these decays.
In 1905, Albert Einstein formulated the idea of mass–energy equivalence. While the work on radioactivity by Becquerel, Pierre and Marie Curie predates this, an explanation of the source of the energy of radioactivity would have to wait for the discovery that the nucleus itself was composed of smaller constituents, the nucleons.
Rutherford's team discovers the nucleus
In 1907 Ernest Rutherford published "Radiation of the α Particle from Radium in passing through Matter."[3] Geiger expanded on this work in a communication to the Royal Society[4] with experiments he and Rutherford had done passing α particles through air, aluminum foil and gold leaf. More work was published in 1909 by Geiger and Marsden[5] and further greatly expanded work was published in 1910 by Geiger,[6] In 1911-2 Rutherford went before the Royal Society to explain the experiments and propound the new theory of the atomic nucleus as we now understand it.
The key experiment behind this announcement happened in 1910 at the University of Manchester, as Ernest Rutherford's team performed a remarkable experiment in which Hans Geiger and Ernest Marsden under his supervision fired alpha particles (helium nuclei) at a thin film of gold foil. The plum pudding model predicted that the alpha particles should come out of the foil with their trajectories being at most slightly bent. Rutherford had the idea to instruct his team to look for something that shocked him to actually observe: a few particles were scattered through large angles, even completely backwards, in some cases. He likened it to firing a bullet at tissue paper and having it bounce off. The discovery, beginning with Rutherford's analysis of the data in 1911, eventually led to the Rutherford model of the atom, in which the atom has a very small, very dense nucleus containing most of its mass, and consisting of heavy positively charged particles with embedded electrons in order to balance out the charge (since the neutron was unknown). As an example, in this model (which is not the modern one) nitrogen-14 consisted of a nucleus with 14 protons and 7 electrons (21 total particles), and the nucleus was surrounded by 7 more orbiting electrons.
The Rutherford model worked quite well until studies of nuclear spin were carried out by Franco Rasetti at the California Institute of Technology in 1929. By 1925 it was known that protons and electrons had a spin of 1/2, and in the Rutherford model of nitrogen-14, 20 of the total 21 nuclear particles should have paired up to cancel each other's spin, and the final odd particle should have left the nucleus with a net spin of 1/2. Rasetti discovered, however, that nitrogen-14 has a spin of 1.
James Chadwick discovers the neutron
In 1932 Chadwick realized that radiation that had been observed by Walther Bothe, Herbert L. Becker, Irène and Frédéric Joliot-Curie was actually due to a neutral particle of about the same mass as the proton, that he called the neutron (following a suggestion about the need for such a particle, by Rutherford). In the same year Dmitri Ivanenko suggested that neutrons were in fact spin 1/2 particles and that the nucleus contained neutrons to explain the mass not due to protons, and that there were no electrons in the nucleus—only protons and neutrons. The neutron spin immediately solved the problem of the spin of nitrogen-14, as the one unpaired proton and one unpaired neutron in this model, each contribute a spin of 1/2 in the same direction, for a final total spin of 1.
With the discovery of the neutron, scientists at last could calculate what fraction of binding energy each nucleus had, from comparing the nuclear mass with that of the protons and neutrons which composed it. Differences between nuclear masses were calculated in this way and—when nuclear reactions were measured—were found to agree with Einstein's calculation of the equivalence of mass and energy to high accuracy (within 1 percent as of in 1934).
Proca's equations of the massive vector boson field
Alexandru Proca was the first to develop and report the massive vector boson field equations and a theory of the mesonic field of nuclear forces. Proca's equations were known to Wolfgang Pauli[7] who mentioned the equations in his Nobel address, and they were also known to Yukawa, Wentzel,Taketani, Sakata,Kemmer,Heitler and Fröhlich who appreciated the content of Proca's equations for developing a theory of the atomic nuclei in Nuclear Physics.[8][9][10][11][12]
Yukawa's meson postulated to bind nuclei
In 1935 Hideki Yukawa proposed the first significant theory of the strong force to explain how the nucleus holds together. In the Yukawa interaction a virtual particle, later called a meson, mediated a force between all nucleons, including protons and neutrons. This force explained why nuclei did not disintegrate under the influence of proton repulsion, and it also gave an explanation of why the attractive strong force had a more limited range than the electromagnetic repulsion between protons. Later, the discovery of the pi meson showed it to have the properties of Yukawa's particle.
With Yukawa's papers, the modern model of the atom was complete. The center of the atom contains a tight ball of neutrons and protons, which is held together by the strong nuclear force, unless it is too large. Unstable nuclei may undergo alpha decay, in which they emit an energetic helium nucleus, or beta decay, in which they eject an electron (or positron). After one of these decays the resultant nucleus may be left in an excited state, and in this case it decays to its ground state by emitting high energy photons (gamma decay).
The study of the strong and weak nuclear forces (the latter explained by Enrico Fermi via Fermi's interaction in 1934) led physicists to collide nuclei and electrons at ever higher energies. This research became the science of particle physics, the crown jewel of which is the standard model of particle physics which describes the strong, weak, and electromagnetic forces.
Modern nuclear physics
A heavy nucleus can contain hundreds of nucleons which means that with some approximation it can be treated as a classical system, rather than a quantum-mechanical one. In the resulting liquid-drop model, the nucleus has an energy which arises partly from surface tension and partly from electrical repulsion of the protons. The liquid-drop model is able to reproduce many features of nuclei, including the general trend of binding energy with respect to mass number, as well as the phenomenon of nuclear fission.
Superimposed on this classical picture, however, are quantum-mechanical effects, which can be described using the nuclear shell model, developed in large part by Maria Goeppert-Mayer. Nuclei with certain numbers of neutrons and protons (the magic numbers 2, 8, 20, 50, 82, 126, ...) are particularly stable, because their shells are filled.
Other more complicated models for the nucleus have also been proposed, such as the interacting boson model, in which pairs of neutrons and protons interact as bosons, analogously to Cooper pairs of electrons.
Much of current research in nuclear physics relates to the study of nuclei under extreme conditions such as high spin and excitation energy. Nuclei may also have extreme shapes (similar to that of Rugby balls) or extreme neutron-to-proton ratios. Experimenters can create such nuclei using artificially induced fusion or nucleon transfer reactions, employing ion beams from an accelerator. Beams with even higher energies can be used to create nuclei at very high temperatures, and there are signs that these experiments have produced a phase transition from normal nuclear matter to a new state, the quark-gluon plasma, in which the quarks mingle with one another, rather than being segregated in triplets as they are in neutrons and protons.
Nuclear decay
Main article:
RadioactivityEighty elements have at least one stable isotope never observed to decay, amounting to a total of about 255 stable isotopes. However, thousands of isotopes have been characterized that are unstable. These radioisotopes decay over time scales ranging from fractions of a second to weeks, years, or billions of years.
The stability of a nucleus is highest when it falls into a certain range or balance of composition of neutrons and protons; too few or too many neutrons may cause it to decay. For example, in beta decay a nitrogen-16 atom (7 protons, 9 neutrons) is converted to an oxygen-16 atom (8 protons, 8 neutrons) within a few seconds of being created. In this decay a neutron in the nitrogen nucleus is converted into a proton and an electron and an antineutrino by the weak nuclear force. The element is transmuted to another element in by acquiring the created proton.
In alpha decay the radioactive element decays by emitting a helium nucleus (2 protons and 2 neutrons), giving another element, plus helium-4. In many cases this process continues through several steps of this kind, including other types of decays, until a stable element is formed.
In gamma decay, a nucleus decays from an excited state into a lower energy state, by emitting a gamma ray. The element is not changed to another element in the process (no nuclear transmutation is involved).
Other more exotic decays are possible (see the main article). For example, in internal conversion decay, the energy from an excited nucleus may be used to eject one of the inner orbital electrons from the atom, in a process which produces high speed electrons, but is not beta decay, and (unlike beta decay) does not transmute one element to another.
Nuclear fusion
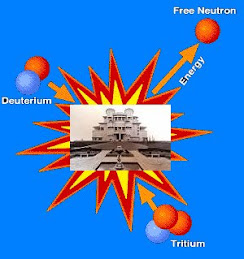
In nuclear fusion, two low mass nuclei come into very close contact with each other, so that the strong force fuses them. It requires a large amount of energy to overcome the repulsion between the nuclei for the strong or nuclear forces to produce this effect, therefore nuclear fusion can only take place at very high temperatures or high pressures. Once the process succeeds, a very large amount of energy is released and the combined nucleus assumes a lower energy level. The binding energy per nucleon increases with mass number up until nickel-62. Stars like the Sun are powered by the fusion of four protons into a helium nucleus, two positrons, and two neutrinos. The uncontrolled fusion of hydrogen into helium is known as thermonuclear runaway. A frontier in current research at various institutions, for example the Joint European Torus (JET) and ITER, is the development of an economically viable method of using energy from a controlled fusion reaction.
Nuclear fission
Nuclear fission is the reverse process of fusion. For nuclei heavier than nickel-62 the binding energy per nucleon decreases with the mass number. It is therefore possible for energy to be released if a heavy nucleus breaks apart into two lighter ones.
The process of alpha decay is in essence a special type of spontaneous nuclear fission. This process produces a highly asymmetrical fission because the four particles which make up the alpha particle are especially tightly bound to each other, making production of this nucleus in fission particularly likely.
For certain of the heaviest nuclei which produce neutrons on fission, and which also easily absorb neutrons to initiate fission, a self-igniting type of neutron-initiated fission can be obtained, in a so-called chain reaction. Chain reactions were known in chemistry before physics, and in fact many familiar processes like fires and chemical explosions are chemical chain reactions. The fission or "nuclear" chain-reaction, using fission-produced neutrons, is the source of energy for nuclear power plants and fission type nuclear bombs, such as those detonated by the United States in Hiroshima and Nagasaki, Japan, at the end of World War II. Heavy nuclei such as uranium and thorium may undergo spontaneous fission, but they are much more likely to undergo decay by alpha decay.
For a neutron-initiated chain-reaction to occur, there must be a critical mass of the element present in a certain space under certain conditions (these conditions slow and conserve neutrons for the reactions). There is one known example of a natural nuclear fission reactor, which was active in two regions of Oklo, Gabon, Africa, over 1.5 billion years ago. Measurements of natural neutrino emission have demonstrated that around half of the heat emanating from the Earth's core results from radioactive decay. However, it is not known if any of this results from fission chain-reactions.
Production of heavy elements
According to the theory, as the Universe cooled after the big bang it eventually became possible for common subatomic particles as we know them (neutrons, protons and electrons) to exist. The most common particles created in the big bang which are still easily observable to us today were protons and electrons (in equal numbers). The protons would eventually form hydrogen atoms. Almost all the neutrons created in the Big Bang were absorbed into helium-4 in the first three minutes after the Big Bang, and this helium accounts for most of the helium in the universe today (see Big Bang nucleosynthesis).
Some fraction of elements beyond helium were created in the Big Bang, as the protons and neutrons collided with each other (lithium, beryllium, and perhaps some boron), but all of the "heavier elements" (heavier than carbon, element number 6) that we see today, were created inside of stars during a series of fusion stages, such as the proton-proton chain, the CNO cycle and the triple-alpha process. Progressively heavier elements are created during the evolution of a star.
Since the binding energy per nucleon peaks around iron, energy is only released in fusion processes occurring below this point. Since the creation of heavier nuclei by fusion costs energy, nature resorts to the process of neutron capture. Neutrons (due to their lack of charge) are readily absorbed by a nucleus. The heavy elements are created by either a slow neutron capture process (the so-called s process) or by the rapid, or r process. The s process occurs in thermally pulsing stars (called AGB, or asymptotic giant branch stars) and takes hundreds to thousands of years to reach the heaviest elements of lead and bismuth. The r process is thought to occur in supernova explosions because the conditions of high temperature, high neutron flux and ejected matter are present. These stellar conditions make the successive neutron captures very fast, involving very neutron-rich species which then beta-decay to heavier elements, especially at the so-called waiting points that correspond to more stable nuclides with closed neutron shells (magic numbers). The r process duration is typically in the range of a few seconds.
Introduction to Nuclear Physics
In general, the nucleus consists of particles called nucleons. There are two types of nucleons: positively charged protons and neutral neutrons . The charge on the proton is negative the charge on the electron:
An atom is therefore neutral when it has the same number of electrons “orbiting” around the nucleus as it has protons in the nucleus. (The neutrons add mass but not charge to the nucleus.) When an atom has more or fewer electrons than the number of protons in the nucleus, then the atom carries a net charge and is said to be an ion.
An element is a substance comprised solely of one type of atom. Carbon, for example, is a substance that consists solely of carbon atoms. What makes a carbon atom different than the atom of another element – say silicon – is the number of protons in its nucleus. All carbon atoms have six protons in their nuclei (plural of nucleus), for example. No matter how many neutrons are in a nucleus and no matter how many electrons orbit the nucleus, if the nucleus contains six protons, then it is a carbon atom. Silicon on the other hand has fourteen protons in its nucleus.
We can see that the number of protons in the nucleus is an important quantity, and is therefore given a special name and a special symbol. The atomic number, Z , of a nucleus is the number of proton charges in that nucleus. (This is usually, but not always, equal to the number of protons. See the last part of Ex. 10.2.) The number of nucleons in a nucleus (the number of protons plus neutrons), A, is called the mass number. The neutron number, N, is the number of neutrons in the nucleus, which is obviously equal to A – Z.
The reason that we can get away with calling a pure number like the number of nucleons a mass number is that, if we multiply the atomic mass by a unit called the atomic mass unit, denoted u, then we get a good approximation for the mass of the given nucleus.
The mass number A also gives us a measure of the molar atomic mass in the sense that the atomic mass is numerically equal to the mass in grams of Avogadro’s number of those nuclei. (Recall that Avogadro’s number is equal to NA = 6.02 x 1023.)
For example, a nitrogen nucleus with A = 14 has a mass number equal to 14. Therefore, one nitrogen nucleus has a mass of 14 u = 2.32 x 10 –26 kg, while one mole of nitrogen nuclei has a mass of 14 g.
Sources:
1. Wikipedia
2. Google
3. http://frank.mtsu.edu/~phys2020/index.htm
 ,
,