In the mathematically rigorous formulation of quantum mechanics developed by Paul Dirac[8] and John von Neumann,[9] the possible states of a quantum mechanical system are represented by unit vectors (called "state vectors"). Formally, these reside in a complex separable Hilbert space (variously called the "state space" or the "associated Hilbert space" of the system) well defined up to a complex number of norm 1 (the phase factor). In other words, the possible states are points in the projective space of a Hilbert space, usually called the complex projective space. The exact nature of this Hilbert space is dependent on the system; for example, the state space for position and momentum states is the space of square-integrable functions, while the state space for the spin of a single proton is just the product of two complex planes. Each observable is represented by a maximally Hermitian (precisely: by a self-adjoint) linear operator acting on the state space. Each eigenstate of an observable corresponds to an eigenvector of the operator, and the associated eigenvalue corresponds to the value of the observable in that eigenstate. If the operator's spectrum is discrete, the observable can only attain those discrete eigenvalues.
In the formalism of quantum mechanics, the state of a system at a given time is described by a complex wave function, also referred to as state vector in a complex vector space.[10] This abstract mathematical object allows for the calculation of probabilities of outcomes of concrete experiments. For example, it allows one to compute the probability of finding an electron in a particular region around the nucleus at a particular time. Contrary to classical mechanics, one can never make simultaneous predictions of conjugate variables, such as position and momentum, with accuracy. For instance, electrons may be considered to be located somewhere within a region of space, but with their exact positions being unknown. Contours of constant probability, often referred to as "clouds", may be drawn around the nucleus of an atom to conceptualize where the electron might be located with the most probability. Heisenberg's uncertainty principle quantifies the inability to precisely locate the particle given its conjugate momentum.[11]
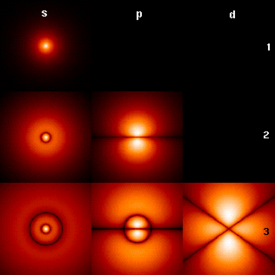 Fig. 1: Probability densities corresponding to thewavefunctions of an electron in a hydrogen atompossessing definite energy levels (increasing from the top of the image to the bottom: n = 1, 2, 3, ...) andangular momentum (increasing across from left to right:s, p, d, ...). Brighter areas correspond to higher probability density in a position measurement. Wavefunctions like these are directly comparable toChladni's figures of acoustic modes of vibration inclassical physics and are indeed modes of oscillation as well: they possess a sharp energy and thus a keenfrequency. The angular momentum and energy arequantized, and only take on discrete values like those shown (as is the case for resonant frequencies in acoustics).
Fig. 1: Probability densities corresponding to thewavefunctions of an electron in a hydrogen atompossessing definite energy levels (increasing from the top of the image to the bottom: n = 1, 2, 3, ...) andangular momentum (increasing across from left to right:s, p, d, ...). Brighter areas correspond to higher probability density in a position measurement. Wavefunctions like these are directly comparable toChladni's figures of acoustic modes of vibration inclassical physics and are indeed modes of oscillation as well: they possess a sharp energy and thus a keenfrequency. The angular momentum and energy arequantized, and only take on discrete values like those shown (as is the case for resonant frequencies in acoustics).Kategori:
- Course material
- Doron Cohen: Lecture notes in Quantum Mechanics (comprehensive, with advanced topics).
- MIT OpenCourseWare: Chemistry.
- MIT OpenCourseWare: Physics. See 8.04
- Stanford Continuing Education PHY 25: Quantum Mechanics by Leonard Susskind, seecourse description Fall 2007
- 5½ Examples in Quantum Mechanics
- Imperial College Quantum Mechanics Course.
- Spark Notes - Quantum Physics.
- Quantum Physics Online : interactive introduction to quantum mechanics (RS applets).
- Experiments to the foundations of quantum physics with single photons.
- Motion Mountain, Volume IV - A modern introduction to quantum theory, with several animations.
- AQME : Advancing Quantum Mechanics for Engineers — by T.Barzso, D.Vasileska and G.Klimeck online learning resource with simulation tools on nanohub
- Quantum Mechanics by Martin Plenio
- Quantum Mechanics by Richard Fitzpatrick
- Online course on Quantum Transport




Tidak ada komentar:
Posting Komentar